JoysBio Coronavirus Covid-19 Antigen Rapid Test Kit EUA 20/Box
JoysBio Coronavirus Antigen Rapid Test Kit
Covid-19 Antigen Nasal Nasopharyngeal Test Cassette Kit, LDT-Skippack Medical EUA
JOYSBIO Biotechnology proudly announced the launching of a new COVID-19 Antigen Rapid Test Kit in June 2020. (Colloidal Gold).
During the acute phase of illness, the novel coronavirus antigen test kit is a lateral flow immunoassay for the qualitative detection of SARS-COV-2 antigen (nucleocapsid protein) in upper respiratory samples obtained from nasal swabs or saliva.
Features:
- Rapid detection in 15 minutes
- Coronavirus antigen test that is simple to use
- Nasal (NS) swab sample collection is less intrusive.
- Versions with a nasopharyngeal swab and saliva are available.
- CE-IVD marked
Performance Characteristics:
Between October 2020 and January 2021, the coronavirus Ag test kit from JOYSBIO was independently validated at Centro Diagnostico Delta S.r.l. in Italy. The COVID-19 Antigen Rapid Test Kit from JOYSBIO was used to test 107 positive specimens.
Nasal swabs were used to obtain samples from patients suspected of having COVID-19. The sensitivity and specificity of the coronavirus antigen test kit are compared to a CE-IVD approved RT-PCR test kit. SARS-CoV is no longer spreading in the population, hence this clinical evaluation is based on that assumption.
The detection sensitivity is 98.13 percent, and the specificity is 99.22 percent, according to a clinical examination of 492 samples.
- Positive Percent Agreement (PPA) = 105/107 (98.13%) (95%CI: 93.4%~99.8%)
- Negative Percent Agreement (NPA) = 382/385 (99.22%) (95%CI:97.7%~99.8%)
- Accuracy = (105+382)/492×100%=98.98%
- Kappa = 2×(105×382-3×2)/(108×385+107 ×384) = 0.97>0.5
This product’s limit of detection (LOD) was calculated using a gradient dilution approach and is 1.6 x 102 TCID50/mL.
Related Scientific Publications
- Five Antigen Tests for SARS-CoV-2: Virus Viability Matters
- Lateral flow antigen tests can sensitively detect live cultured virus of the SARS-CoV-2 B1.1.7 lineage
- Impaired performance of SARS-CoV-2 antigen-detecting rapid tests at elevated temperatures
- Limited specificity of SARS-CoV-2 antigen-detecting rapid diagnostic tests at low temperatures
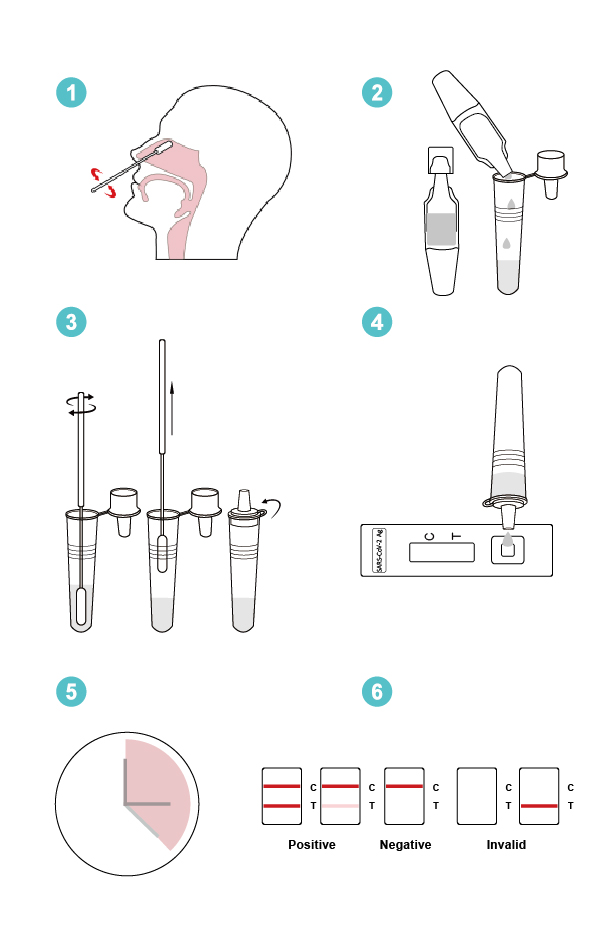
COVID-19 Antigen Rapid Test Principle
The coronavirus antigen fast test kit is a lateral flow assay that detects nucleocapsid (N) protein in upper respiratory samples in a qualitative manner (nasal swabs). The sandwich immunoassay format was used to create this lateral flow assay.
Coronavirus N protein binds with colloidal gold-labeled SARS-CoV-2 N protein antibody to produce an antibody-antigen (Ab-Ag) complex when the specimen is placed on the sample pad of a test cassette.
SARS-CoV-2 N protein antibody (Rabbit monoclonal antibody) captures the Ab-Ag complex as it migrates to the test line under capillary action.
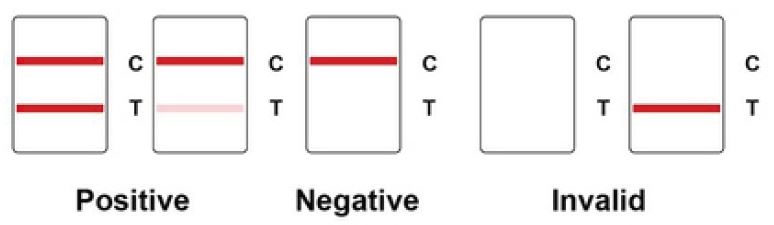
A red band will appear on the test line, indicating that the specimen is positive for COVID-19 nucleocapsid protein.
If the material does not include any coronavirus antigen (N protein) or the antigen level is below the detection limit, no color band will emerge on the test line.
Call 440-463-4480 For Availability